What is a Bubble Study (or a PFO Test)?
PFO is a hole between the left and right atriums of the heart, which creates a right to left shunt for blood flow. This hole typically exists before birth, but it most often closes shortly after birth. If the hole fails to close naturally after birth, it is referred to as a Patent Foramen Ovale (PFO).
Embolism through a Patent Foramen Ovale (PFO), can be a significant cause of stroke in young adults. Transcranial Doppler is one of the preferred modalities for quick and non-invasive identification and assessment of PFO, also known as a “Right-to-Left Shunt”.
The PFO test with TCD is often called a “bubble test”, “bubble study”, or “agitated saline” because it involves the intravenous injection of micro-bubbles (air mixed saline). Along with the injection, the Doppler signal is recorded with a TCD system, most often in the Middle Cerebral artery (MCA).
In the event of an existing Patent Foramen Ovale, the air bubbles that are actually microemboli pass through the hole from the right heart to the left side through the open shunt. Therefore, the blood which is pumped out of the heart includes a mix of these air microemboli and reaches the cerebral circulation.
Clinical Significance of PFO Diagnosis
PFO is a hole between the left atrium and the right atrium of the heart. This hole between the chambers of the heart normally closes naturally soon after birth, but in a large percentage of the population, it fails to close and is referred to as a Patent Foramen Ovale (PFO).
The main risk factors associated with this condition are that blood clots generated in the venous circulation reach the upper chambers of the heart. From these two upper chambers, the blood clot may reach the brain through the blood flow. TCD is the best choice to diagnose a PFO.
How to use TCD for the PFO (Bubbles Test)
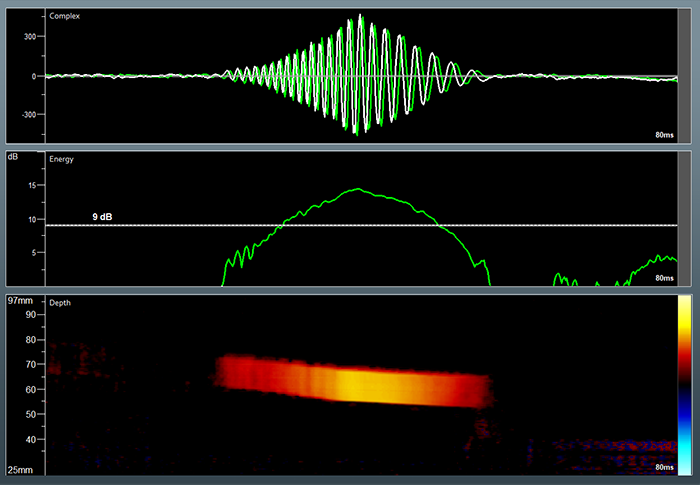
During bubble study, the spectral Doppler easily detects air bubbles that pass through the cerebral arterial circulation (such as the MCA). Such air bubbles have the characteristics of High-Intensity Transient Signals (HITS) with distinct increased signal energy.
To help direct the blood towards the cerebral circulation and maximize the effect of the bubble test, the patient is asked to perform the Valsalva maneuver during the test. The total emboli counted during the test is a clear indication of whether PFO exists and, if it exists, how severe it is.
Performing Bubble Studies with TCD Robotic Probe
Conventional TCD devices perform bilateral studies via the standard cumbersome monitoring headsets. This approach results in a time-consuming fixation process that may often cause frustration to both technicians and patients. In addition, the headset assembly process requires an experienced and professional neuro-sonologist, physician, or technologist. As a result, sonographers often prefer to avoid using the standard TCD headsets and instead, with no reasonable alternative, prefer to hold the probe manually and perform a unilateral study.
Bubble studies done with regular headsets introduce several problems:
- It normally requires more than one person to perform the PFO study.
- It is normally used only on one side of the brain, skipping important bilateral PFO information for improved confirmation of right to left shunt size and better clinical diagnosis.
- Holding the probe manually for a prolonged period of time can be difficult, and the signal may get lost.
- Assembly of the standard monitoring headgears can consume a significant amount of time.
Modern Robotic TCD probes were invented to solve these issues and improve the quality of the bubble tests. If an adequate acoustic window exists, the sonographer can quickly and automatically find a Doppler signal within a matter of seconds. The automatic TCD robotic monitoring method eliminates the cumbersome need to fix probes with regular TCD headgears. Most importantly, a robotic TCD probe improves the quality of the bubble study test results by detecting Micro-Embolic Signals on both sides of the brain and reducing errors caused by accidental probe movements.

2 MHz Doppler Probe
High quality and ultra sensitive Doppler probe

Monitoring Headset
Perform PFO Test Bilaterally

Robotic Probe
Fast, Stable, and Comfortable Monitoring
Using Dolphin for PFO Test
The Dolphin TCD Equipment has a dedicated PFO protocol designed to complete the test rapidly per all clinical guidelines. The PFO protocol supports both a unilateral examination or a more complete assessment, a bilateral examination using the Dolphin/XF Robotic Headframe.
For improved HITS identification, the Dolphin supports a Power M Mode display, which helps visualize the high-intensity bubbles that pass through the target vessel. More importantly, the Dolphin allows measuring the total HITS count not only from the main selected depth but also all of the HITS identified at all depths along the ultrasound beam. This unique feature allows measuring the suspected emboli counts in a single measurement, for example, in the MCA and ACA arteries.
Frequently, the examination calls for performing several measurement sessions, such as with/without the Valsalva maneuver, which can take place before or after the bubble injection. The Dolphin PFO protocol allows performing multiple examination sessions in a single continuous measurement. Each session is given its own name, with automatic cursors placed to mark the different stages of the examination. A separate automatic HITS count is performed for each session, as well as a series of additional clinical information that is provided for optimal PFO diagnosis.
The Dolphin HITS analysis has an unmatched temporal resolution, which allows for digging deep and counting HITS during the embolic shower. A sophisticated HITS analysis program allows a detailed investigation of each suspected embolic event, which includes the energy and motion in the artery of each event, a high-resolution embolic m-mode display, and more.
Expected Results of a Bubble Study
There are several grading criteria to characterize the PFO or the right-to-left shunt, based on the count of the Micro-Embolic Signals (MES).
The criteria by the ICC (International Consensus Criteria) is as follows:
| Grade | MES Count |
|---|---|
| 0 | None |
| 1 | 1-10 |
| 2 | 11-30 |
| 3 | > 30, with embolic shower |
Another grading scale is referred to as Spencer’s Logarithmic Scale:
| Grade | MES Count |
|---|---|
| 0 | None |
| 1 | 1-10 |
| 2 | 11-30 |
| 3 | 31-100 |
| 4 | 101-300 |
| 5 | > 300 |
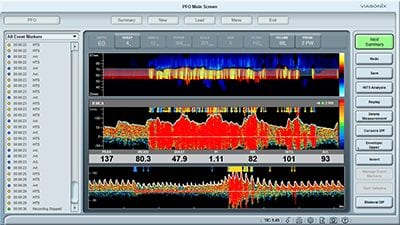
An example of a PFO test performed with Viasonix Dolphin. The lower spectrum region presents the entire test for a quick review.
Selected Literature
Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Sloan MA et al., Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004 May 11;62(9):1468-81
Transcranial Doppler: Techniques and advanced applications: Part 2, Sharma AK, Bathala L, Batra A, Mehndiratta MM, Sharma VK, Ann Indian Acad Neurol. 2016 Jan-Mar;19(1):102-7
Transcranial doppler ultrasonography should it be the first choice for persistent foramen ovale screening?, Monika Komar et al., Cardiovascular Ultrasound 2014, 12:16
Diagnostic Criteria for Cerebrovascular Ultrasound, Georgios Tsivgoulis, Marsha M. Neumyer & Andrei V. Alexandrov, In Cerebrovascular Ultrasound in Stroke Prevention and Treatment, Edited by Andrei V. Alexandrov, 2011 Blackwell Publishing Ltd., Chapter 6
Transcranial Doppler Ultrasound: Incremental Diagnostic Role in Cryptogenic Stroke Part II, Antonello D’Andrea et al., J Cardiovasc Echogr. 2016 Jul-Sep; 26(3): 71–77
Diagnosis of Patent Foramen Ovale: The Combination of Contrast Transcranial Doppler, Contrast Transthoracic Echocardiography, and Contrast Transesophageal Echocardiography, Xiaoxue Yang et al., BioMed Research International, Volume 2020, Article ID 8701759, 7 pages
Disclaimer of Information & Content
The content of Viasonix Ltd. website is for information only, not advice or guarantee of outcome. Information is gathered and shared from reputable sources; however, Viasonix Ltd. Management is not responsible for errors or omissions in reporting or explanation. No individuals, including those under our active care, should use the information, resources or tools contained within this self-diagnosis or self-treat any health-related condition. Viasonix Ltd. Management gives no assurance or warranty regarding the accuracy, timeliness or applicability or the content.