What is Pulse Volume Recording?
Pulse Volume Recording (PVR) is a pneumo-plethysmography test that is widely used for non-invasive diagnosis of lower extremity peripheral arterial disease (PAD).
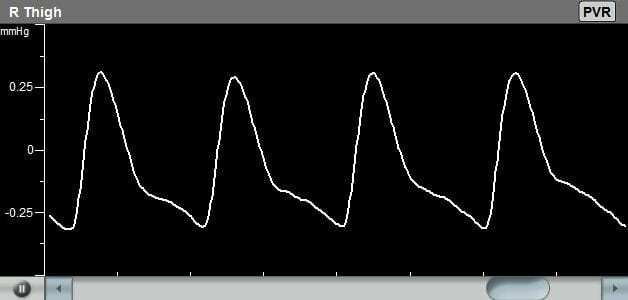
Pulse Volume Recording is an air plethysmography waveform analysis test used to detect the segmental volume changes in the limb resulting from the flowing blood as a function of the cardiac cycle. This test is usually performed with a PVR machine, also known as a Plethysmograph.
PVR tests are quick, reliable and painless, and can provide valuable information to help diagnose and treat PAD.
How to Perform PVR Measurements
Performing a Pulse Volume Recording (PVR) test is a crucial step in diagnosing lower extremity peripheral arterial disease (PAD) using the PVR machine.
Follow these steps to perform PVR test:
- Make sure the patient is relaxed and in a comfortable position.
- Similar to the Segmental Blood Pressure (SBP) measurements, wrap dedicated pressure cuffs around the target limb segments prior to initiating the test. These sites typically include the thigh, above the knee, below the knee, and ankle on each leg.
- Set the PVR target pressure to a level that would occlude the venous return yet will not obstruct the arterial flow. This target pressure is typically 65 mmHg and is kept constant throughout the PVR test.
- Inflate the cuffs using a PVR machine to the selected pressure.
- Once the cuff pressure is stabilized, the Pulse Volume Recording waveform is generated based on the pressure changes in the air inside the pressure cuff as the result of minute limb circumference changes.
- Interpret the test results. The elasticity or stiffness of the arterial circulation in the legs will determine, in part, the shape of the PVR waves. This test is mostly qualitative in nature, and the focus is on the shape of the PVR-obtained waveforms.
This step-by-step guide for performing a Pulse Volume Recording test is for informational purposes only. Healthcare professionals should rely on their expertise, clinical judgment, and institutional protocols for accurate administration and interpretation. Additional clinical information, patient history, physical examination, and other diagnostic tests may be necessary for comprehensive evaluation.
Using the Falcon for PVR Measurements
As a PVR machine, the Falcon allows immediate Pulse Volume Recording testing that can be completed very rapidly for each measured site.
- Rapid and Precise Measurements: Complete a PVR testing in just a few minutes, minimizing patient wait times.
- Simultaneous Multi-Site Testing: Perform PVR measurements sequentially or simultaneously on up to 10 sites, enabling comprehensive evaluations in a single test session.
- Intuitive User Interface: Easily adjust the preferred target inflation pressure, sweep time display, scale, or filter.
- Customizable Protocols: Edit PVR protocols to match specific patient conditions and requirements.
- Effortless Testing Process: Simply place color-coded pressure cuffs on the target sites and selecting the desired PVR protocol.
- Comprehensive Analysis: The Falcon provides not only qualitative but also quantitative insights into vascular conditions such as waveform amplitude, rise time, heart rate, RAR, and waveform slope.
- Advanced Features: Such immediate display of contralateral results and superposition of right and left waveforms.
Expected Results of Pulse Volume Recording Test
The diagnosis of the Pulse Volume Recording waveforms is mostly qualitative in nature. Clinicians are normally interested in the qualitative shape of the PVR waveform, specifically the systolic rise curve, the peak amplitude shape, the dicrotic notch during the downward section, and the diastolic section of the waveform.
- Normal waveform: A normal PVR waveform has a rapid upstroke, well-defined peak, prominent dicrotic notch, and gradual downstroke. This suggests normal blood flow and vascular function.
- Reduced blood flow: If the waveform has a delayed or diminished upstroke, flattened or decreased peak, and/or rapid downstroke, it may indicate reduced blood flow due to arterial occlusion, stenosis, or poor vascular compliance.
- Severe peripheral arterial disease: A severely abnormal waveform, such as one with an absent or delayed dicrotic notch, or when the waveform amplitude decreases and ultimately becomes flat, may indicate advanced peripheral arterial disease or poor cardiac function.
Additional quantitative parameters such as the absolute PVR amplitude or the relative amplitude reduction (RAR) and slope are discussed in the literature as possible diagnostic measures.
Selected Literature
Lower Extremity PAD, Hirsch et al. 2005, ACC/AHA 2005 Guidelines for the Management of Patients With Peripheral Arterial Disease
Overview of Peripheral Arterial Disease of the Lower Extremity, Ali F. AbuRahma and John E. Campbell, Noninvasive Vascular Diagnosis, A.F. AbuRahma (ed.), Springer International Publishing AG 2017, Ch 21, pp 291-318
Objective lower extremity arterial plethysmographic waveform characteristics for differentiating significant inflow disease in nondiabetic patients, Robert Scissons, Journal of Diagnostic Medical Sonography, I6, 2014
Noninvasive Physiologic Vascular Studies: A Guide to Diagnosing Peripheral Arterial Disease, Robert Sibley et al, radiographics.rsna.org, Volume 37 Number 1, p 346-357.
2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease; A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines; Journal of the American College of Cardiology, Vol. 69, No. 11, 2017
Lower Extremity Arterial Physiologic Evaluations, Vascular Technology Professional Performance Guidelines, the Society for Vascular Ultrasound, 2019
Disclaimer of Information & Content
The content of Viasonix Ltd. website is for information only, not advice or guarantee of outcome. Information is gathered and shared from reputable sources; however, Viasonix Ltd. Management is not responsible for errors or omissions in reporting or explanation. No individuals, including those under our active care, should use the information, resources or tools contained within this self-diagnosis or self-treat any health-related condition. Viasonix Ltd. Management gives no assurance or warranty regarding the accuracy, timeliness or applicability or the content.