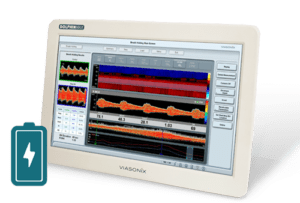
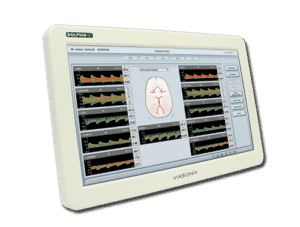
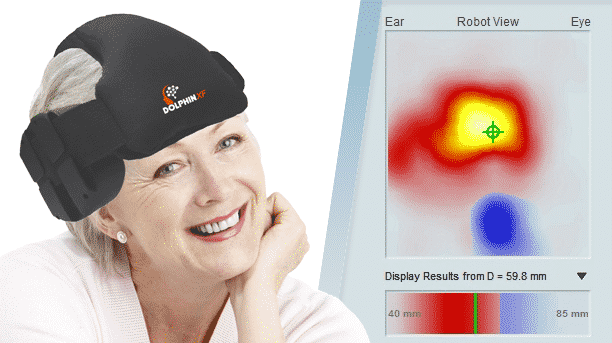
Viasonix is proud to announce the reception of FDA approval and the launch of its IP-protected Dolphin/XF, a new Transcranial Doppler (TCD) Robot with sophisticated algorithms that will dramatically impact the TCD market. The Dolphin/XF Robot connects to any of the advanced Dolphin TCD models to automatically assist the users in quickly obtaining bilateral cerebral blood flow velocities for the purpose of short- or long-term monitoring.
The combination of the unique online and offline processing capabilities of the Dolphin TCD machines and the powerful scanning abilities of the Dolphin/XF robotic probe allows for fast and effective scanning of the brain. Unlike other attempts in the marketplace, the Dolphin/XF is not bulky, is clinically effective, is cost-effective, and is practical for use in various critical care settings such as the OR and the ICU, as well as in other diagnosis TCD labs.
“We are very excited to finally launch the Dolphin/XF TCD robot”, said Dr. Dan Manor, President, and CEO of Viasonix. “Our revolutionary robot is a significant breakthrough in brain diagnosis in the neuro-clinical market. It will assist the medical staff in reducing the setup time for bilateral monitoring and replace other bulky and time-consuming solutions that are currently in the TCD market. Our design was focused on a simple and quick operation to obtain bilateral waveforms in a matter of seconds”.
Dr. Manor also added: “We designed the Dolphin/XF to be affordable to all TCD users and reach all markets with a cost-effective low price. Coupled with the Dolphin Transcranial Doppler systems, which are already considered by many to be the best TCD machines in the market, the Dolphin/XF provides new horizons for brain diagnosis, particularly in critical care settings such as the Operating Room (OR), Intensive Care Unit (ICU), or Emergency Room (ER). The international demand for the Dolphin/XF has already significantly exceeded our expectations. Leading medical centers across the world are excited to use our new TCD robot.
Currently, we already have a backorder log, but we are working hard to decrease the waiting time and meet the increasing demand”.
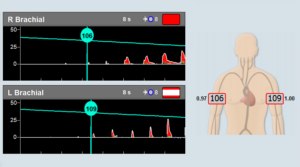
The Dolphin/XF bilateral TCD robot fits very comfortably on the head. The patient can either lie down, sit upright, stand up, or even walk (with the battery-operated Dolphin/MAX system) during the monitoring session. The Dolphin/XF can monitor all types of cerebral blood vessels at any selected depth. This capability is particularly ideal for monitoring stroke or vasospasm patients, patients with stroke threatening emboli, critical care patients with unstable Intracranial Pressure (ICP), TCD monitoring during anaesthesia, or even standard routine examinations such as Patent Foramen Ovale (PFO), Vasomotor Reactivity Tests (VMR) or the Breath Holding Test (BH). While monitoring, the users can enjoy all the other advanced monitoring features supported by the Dolphin TCD device. Viasonix is currently shipping the Dolphin/XF internationally to many locations across the world.
We invite you to contact us for more information and a demonstration.
This press release also appeared on: The Chicago News Journal, Boston Herald, Healthcare PR News, International Business Times, Las Vegas News Journal, 24-7 News, Science and Research News, Washington News Journal